
Our HOMED are committed to the research and development of innovative medical technologies and equipment with independent R&D capabilities in respiratory therapy products. We have obtained the US 510K certification, European CE-MDR certification, and ISO 13485 certification, providing ODM services to global customers.

As a deep cultivator in the field of medical devices, we deeply understand that technology is the soul of products. The company has a strong core R&D team, focusing on the technological research and innovation of high-end medical equipment, covering multiple fields such as in vitro diagnosis, nasal care, home nebulization, intensive care, medical consumables, and respiratory rehabilitation. It can accurately perceive market demand and transform clinical pain points into innovative product solutions.
With specialized and high-precision medical device exclusive mold factory, as well as precision machining equipment and testing instruments, we create an automated and intelligent mold production system to ensure quality assurance and safeguard respiratory health.

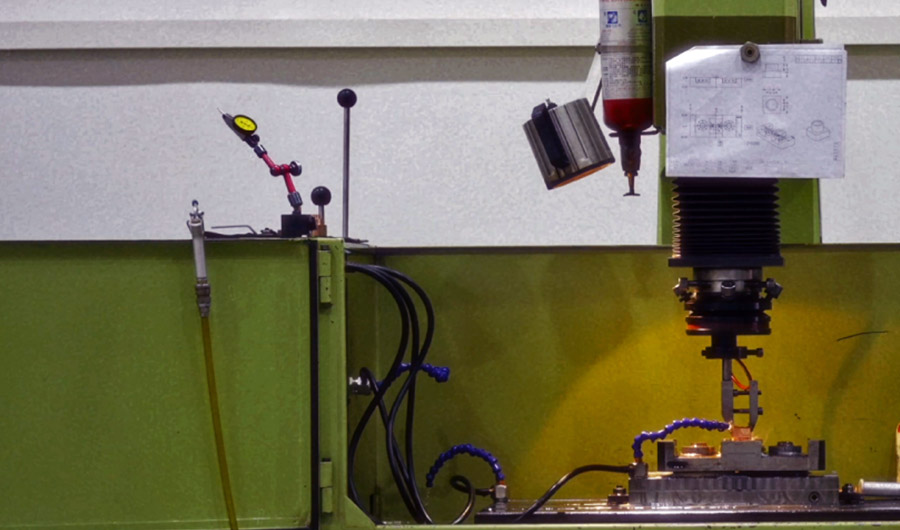


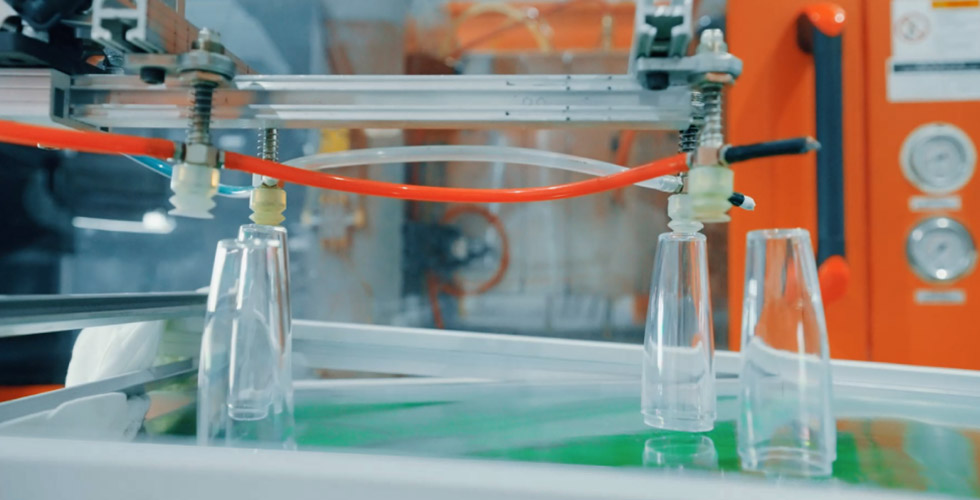
Capable of designing and manufacturing/repairing horizontal tools, as well as process debugging, to achieve efficient automated production which can meet the production needs from precision small parts to large shell parts.
Quality based, equipped with NGI professional R&D experimental center



















